3-Acetoxypropyltrimethoxy silane
| Chemical Name: | 3-Acetoxypropyltrimethoxy silane |
| Alias: | 3-(trimethoxysilyl)propyl acetate |
| Product Category: | CAS 54004-18-1 Acyloxy Silanes | Silanes |
| CAS NO.: | 59004-18-1 |
| Formula: | CH3COO(CH2)Si(OC2H5)3 |
| EINECS: | 261-552-8 |
| Molecular Weight: | 222.31 |
| Molecular Formula: | 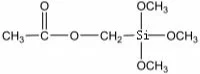 |
| Boiling Point: | 214°C |
| Density: | 1.062±0.005 (ρ20)g/cm3 |
3-Acetoxypropyltrimethoxy silane Description
3-Acetoxypropyltrimethoxy silane is an acetoxy silane, which all have an acetoxy functional group containing an unsaturated double-bond structure and three hydrolyzable alkoxy (methoxy) groups in the molecular structure, and this dual reactivity allows them to improve the bonding and compatibility between the two through bi-directional chemical reactions with both inorganic materials (glass, metal, fillers) and organic polymers (thermosetting resins, plastics, This dual reactivity allows them to improve the bonding, adhesion and compatibility between inorganic materials (glass, metal, filler) and organic polymers (thermosetting resins, plastics, elastomers) through a two-way chemical reaction with the two, thus improving the mechanical properties of resin-based composites, or improving the adhesive strength of resin coatings and water resistance and other properties.
The product can also be copolymerized with monomers such as acrylic acid, methacrylic acid, vinyl acetate, styrene, etc. containing unsaturated double bonds, thereby imparting moisture curing, good adhesion, water resistance, etc. to the copolymer. In different applications, they can be used as coupling agents, adhesion promoters, cross-linking agents, surface modifiers for pigment fillers, silicone-modified graft copolymerization monomers, and so on.
The product can also be copolymerized with monomers such as acrylic acid, methacrylic acid, vinyl acetate, styrene, etc. containing unsaturated double bonds, thereby imparting moisture curing, good adhesion, water resistance, etc. to the copolymer. In different applications, they can be used as coupling agents, adhesion promoters, cross-linking agents, surface modifiers for pigment fillers, silicone-modified graft copolymerization monomers, and so on.
| Product Name | 3-Acetoxypropyltrimethoxy silane |
| Synonyms | 3-(trimethoxysilyl)propyl acetate |
| Boiling point | 214°C |
| Density | 1.062±0.005 (ρ20)g/cm3 |
| Refractive index | 1.415±0.005 (n25D) |
| Appearance | Colorless Transparent liquid, soluble in a variety of organic solvents |
| Package | 5kg/drum, 20kg/ctn. Sealed and stored in a cool, dry and ventilated place, moisture-proof and water-proof, away from fire and heat source. |
| Usage |
|
Packaging Specifications


Jessica G.
Get in touch to Get
- Quick and helpful reply within 8 hours;
- Tailored solutions provided for your project;
- One-stop purchasing service.
3-Acetoxypropyltrimethoxy silane: Guide
The hydrolysis of 3-Acetoxypropyltrimethoxy silane requires the use of organic acids (e.g., formic acid, acetic acid, etc.) as a catalyst, specifically by adjusting the pH of the water to about 3.5-4.5, and then adding CAS 59004-18-1silane and stirring for a period of time (at least 30 minutes or more) until the silane is completely dissolved and the solution is clear and transparent.
Their hydrolysates are unstable and it is recommended to use them up within 24 hours. Fogging of the solution indicates that the silane has partially self-polymerized to form a polymer of silane (silicone) and become ineffective.
Their hydrolysates are unstable and it is recommended to use them up within 24 hours. Fogging of the solution indicates that the silane has partially self-polymerized to form a polymer of silane (silicone) and become ineffective.