3-Chloropropyltrichlorosilane
| Chemical Name: | 3-Chloropropyltrichlorosilane |
| Alias: | γ-chloropropyltrichlorosilane |
| Product Category: | CAS 2550-06-3-5 Chloropropyl Silane | Silanes |
| Structural Formula: | 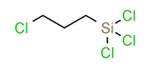 |
| CAS No.: | 2550-06-3 |
| EINECS: | 219-844-8 |
| Molecular Formula: | C3H6Cl4Si |
| Molecular Weight: | 211.977 |
3-Chloropropyltrichlorosilane Description
3-Chloropropyltrichlorosilane (CAS 2550-06-3) is a colorless and transparent liquid, with it as the main raw material can produce chloropropyltriethyl(methyl)oxysilane, aminopropyltriethyl(methyl)oxysilane, bis(triethoxysilylpropyl)tetrasulphide, and other silane coupling agent products, it is an important additive for rubber products, glass fiber reinforced materials. It is an important auxiliary in rubber products and glass fiber reinforcing materials.
| Chemical name | 3-Chloropropyltrichlorosilane |
| Synonymous | EINECS 219-844-8, (γ-Chloropropyl)trichlorosilane, Trichloro(3-chloropropyl)silane, 3-chloropropyl-1-trichlorosilane Silane, G-CHLOROPROPYLTRICHLOROSILANE, 3-Trichlorosilyl-1-Chloropropane, Trichloro-3-chloropropylsilane |
| Density | 1.35 g/mL at 25 °C(lit.) |
| Boiling Point | 181 °C |
| Flash Point | 84°C |
| Vapor Pressure | 1.35mmHg at 25°C |
| Refractive Index | 1.465 |
| Storage Conditions | Inert atmosphere,Room Temperature |
| Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Exterior | clear liquid |
| Proportion | 1.35 |
| Color | Colorless to Almost colorless |
| Brn | 1737851 |
| Packaging |
|
| Sample |
|
| Inventory items |
|
| Price |
|
Packaging Specifications


Jessica G.
Get in touch to Get
- Quick and helpful reply within 8 hours;
- Tailored solutions provided for your project;
- One-stop purchasing service.
3-Chloropropyltrichlorosilane : Guide
3-Chloropropyltrichlorosilane has an irritating odor and is the basic monomer of silane coupling agent. Hydrolysis releases hydrogen chloride and generates the corresponding condensate. The vapor and liquid can cause skin burns, inhalation is toxic. Reacts with anhydrous ethanol, releasing hydrogen chloride and generating 3-chloropropyltriethoxysilane.
The chlorine atom in the C-Cl bond within the molecule can be replaced by hydroxyl, amino, and carboxyl groups through classical organic chemical reactions. It can be produced by reacting trichlorosilane with allyl chloride in the presence of a platinum catalyst. It can also be produced by reacting propyltrichlorosilane with thionyl dichloride in the presence of benzoyl peroxide catalyst. Used to synthesize silicone intermediates, silane coupling agents and polymers.
The chlorine atom in the C-Cl bond within the molecule can be replaced by hydroxyl, amino, and carboxyl groups through classical organic chemical reactions. It can be produced by reacting trichlorosilane with allyl chloride in the presence of a platinum catalyst. It can also be produced by reacting propyltrichlorosilane with thionyl dichloride in the presence of benzoyl peroxide catalyst. Used to synthesize silicone intermediates, silane coupling agents and polymers.